Degreasing is a fundamental operation to prevent any problems of corrosion or stains during pickling, polishing, satin finishing treatments. Residues of oils or fats can in fact alter the efficiency of a pickling bath, but also delimit areas of lower electrolytic conductivity at the metal / solution interphase during an electrolytic treatment. Degreasing can be carried out with organic solvents using the chemical principle “the like dissolves the similar” or with alkaline or acid solvents on a water basis. In this second case, the hydrophilic part of the compound is acted on. For many years, Ricerca Chimica has been promoting a campaign to replace traditional chlorinated solvents for two reasons: the intrinsic harmfulness of classic products and the potential harmfulness of chlorinated molecules on certain types of products. In this sense, the Chemical Research efforts have materialized in the Green Line degreasing division, where a wide range of products free of fluorinated, nitrated and chlorinated species is offered for every type of need. Efforts have been directed towards the use of natural organic acids to guarantee not only degradability but also a low toxicological impact.
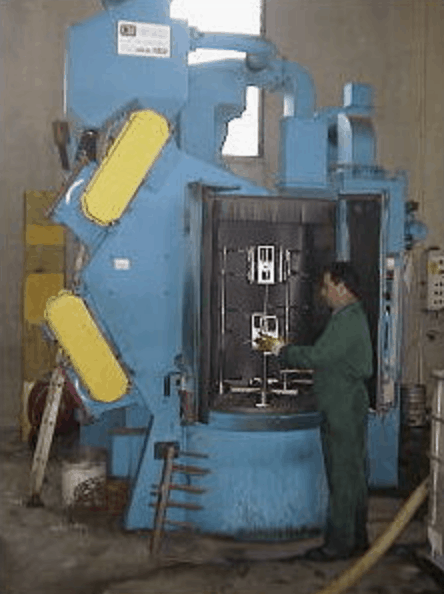
By pickling we mean a chemical operation designed to remove and dissolve surface oxides present on the pieces, such as rust on iron, polluting slags, welding oxides or annealing oxides. This chemical operation is carried out for the majority of cases in immersion processes using strong inorganic acids (sulfuric, nitric, hydrochloric acid) diluted in water. The tanks that are used are normally built or covered in antacid material resistant to the type of compound used. Pickling also allows to remove the chromium depleted steel layer due to the alterations suffered by the material during the treatment (decromised layer). If the welding operations deplete the chromium steel to the point of falling below the reference content of 12%, the material loses its characteristics of stainless. The pickling treatment acts chemically, but also mechanically due to the effect of the hydrogen bubbles developed during the process, which act on iron oxides (wustite, magnetite, hematite). Research Chemistry has long since replaced the traditional nitric / hydrofluoric pickling process with the eco-compatible one free of nitric acid. The absence of NOx emissions into the atmosphere has not only reduced pollution, but has also made the operator’s work less dangerous. The attention of the company is today mainly directed towards the protection of the worker, for this reason the birth of the Green Line we believe is destined to completely and shortly supplant traditional treatments. Ricerca Chimica offers a pickling treatment on austenitic, ferritic, martensitic, inconel, hastelloy, stainless steel and many other materials, with attention to copper and aluminum alloys.

Passivation is a very important treatment because it allows you to restore a steel product to its original characteristics of stainless. Immersion in passivating baths allows not only to extend the “life of the product”, but also to remove any traces of pollutant due to previous processes (especially mechanical). For this reason the passivation treatment is often called “decontamination”. Chromium alloys such as stainless steels are chosen almost exclusively for applications in fields involving corrosive environments. These steels are selected, in particular, for their ability to resist chemical attack without major additional protection costs. This resistance derives from the unique and inimitable structure of the alloy surface. The surface exposed to the corrosive environment is covered with a layer of passive film, which is simply made up of chromium oxide, which in turn is an integral part of the alloy itself. Although this film is formed and reforms rapidly, it is very sensitive to contaminants (metal residues deriving from production / manufacturing treatments, dirt, dust). These residues can start the aggressive attack on the manufactured item in question and cause its technological properties to decline.

Electropolishing is an electrochemical process for removing metal material; in a sense it can be considered as the opposite technique of electro-deposition. As soon as the circuit is closed, the current begins to circulate in the system and an anodic film is deposited on the metal surface. Under particular conditions, this anodic film is believed to be responsible for leveling and polishing the surface. The type and nature of this film and the operating conditions determine whether polishing and leveling occur simultaneously or independently of one another; it is indeed important to remember that one does not imply the other. In addition to the anodic film there are other factors that influence the final result. These include the mechanical agitation of the system by blowing air or rotating the anode, heating the system (with thermostatic temperature control), the density of current applied (or voltage). As the voltage increases, the current density also increases, up to a critical point, where the current decreases sharply, while the potential difference continues to grow (first passivation potential). Beyond this point, the current remains almost constant as the potential increases. We speak of “passivation stage”, in which the material is covered with an oxide layer that protects it from the corrosive attack of the anode current. The extent of this range in which the current remains constant is variable according to the operating conditions and the material, when the potential reaches the critical trans-passivation value, the current starts to grow proportionally to the voltage, subsequently reaching a limit value in which the gas development becomes predominant with respect to metal dissolution. Electropolishing takes place only when the current density is higher than that which occurs at the critical point (current corresponding to the potential of first passivation). Most electropolishing techniques use direct current, although numerous recently published articles seem to support the use of alternating current under particular conditions. The values usually used are 1-10 A / cm2.

Ricerca Chimica INOX SERVICE is able to carry out mechanical polishing processes both manually and through the use of automated equipment. The final result is subjected to rigorous controls that guarantee the high quality standard of our processes.
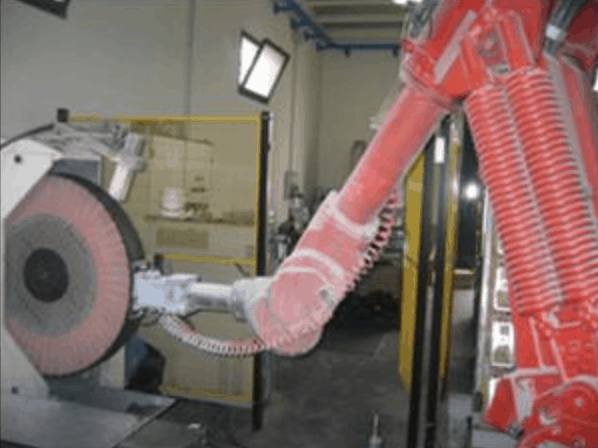
The mechanical shot blasting treatment allows to obtain particular surface finishes with a pleasant aesthetic appearance. ShotBlasted and/or shot-blasted products have a high roughness particularly sought after in architectural and modern design environments.